Make observation of excited state gases.
Orange gas (Neon): four different multi colored slots around the machine. Yellow orange and red are very brightly colored.
Line secptra: Alot more lines then He.
white gas (He): the different color slots are spread further apart on the top and bottom. The left colored slot seems to have two separate slots of colors. Very dull colors, yellow at the end is the brightest. Rainbow on top- colors dull with yellow standing out
Line secptra's- show fewer lines.
Purple gas (Ar): Far away do not see anything- close, dark purple color then rest of slots, the colors are all horizontal with a gap between each color.
Line secptra: very little amount of lines
Light purple gas(Nitrogen): see more shades of colors. Red, orange, yellow, and green stand out. Top slot of colors are smooched together making a rainbow instead of the colors being in separate slots.
Line secptra: Few lines- with reds, oranges, yellows, and greens standing out.
White/Blue gas (Carbon Dioxide): Bright green and yellow- rest of the colors are very dull within the color slots. Top slot of colors make a rainbow, the colors are not divided.
Line secptra: very little amount of lines are shown brightly.
Visualizing Chemistry
Thursday, January 20, 2011
Activity 2 1/20/11 Antiacids
Write the formula for Tums? How does Tums chemically react with water and stomach acid?
CaCO3--> Ca+2 + CO3-2
CO3-2 + H+ --> HCO3- + H+ --> H2CO3 <---> H2O + CO2
CaCO3--> Ca+2 + CO3-2
CO3-2 + H+ --> HCO3- + H+ --> H2CO3 <---> H2O + CO2
How many Tums are needed to neutralize a can of coke?
I tried Tums to neutralize a shot of lemon juice and it took 3 Tums to get it at a pH level of 7. I understand that coke does not have the pH level as lemon juice but I believe it would take probably around 5-7 tums to neutralize a whole can of coke.
I tried Tums to neutralize a shot of lemon juice and it took 3 Tums to get it at a pH level of 7. I understand that coke does not have the pH level as lemon juice but I believe it would take probably around 5-7 tums to neutralize a whole can of coke.
Activity 1 1/20/11 Experimental Design and Conclusions
Reflect on the expansion of water and salt water experiments you and your classmates performed. What are some interesting results and struggles with this experiment.
One of my interesting results from measuring the different heights the water expanded to was that with 1-1/2 tsp's of salt the water expanded more then the cup that had 3 tsp's of salt mixed in the water. It seemed as if the more salt I was going to add to the water the less that it was going to expand when frozen.
I also noticed that it was difficult to measure the different heights that the water's expanded. I did my experiment in clear cups so it was easier to measure the different heights of the frozen water.
Another interesting result I noticed was that it took my cup of water only an hour to completely freeze, while it took my cup with 1-1/2 tsp's of salt about 3 hours, and my 3 tsp's of salt cup took about 4-1/2 hours to freeze.
Relate your thought on this experiment to the scientific question, "is the earth warming?"
"Is the earth warming?" From conducting my experiment with how salt water freezes compared to just tap water, I found that over time temperatures change due to factors that took part in the water, which would be the salt."Just as weather patterns change from day to day, the climate changes too. This occurs naturally, driven by internal and external factors. Through widespread use of land, use of fossil fuels and the building of cities, we have changed our climate." http://www.global-greenhouse-warming.com/climate.html
I was adding a factor to the water which caused the water to take longer to freeze. The more factors we pull into a situation the more "trouble/problems" we may have with the temperatures.
Stated above just shows how with different factors temperature/climate can change. The salt interfered with how fast the water would freeze and just how cold this water would get before it freezes. This is the same for the earth, with cars driving around using fossil fuels and building new cities/buildings also change the temperature and climate on earth. "Temperatures across the globe are most certainly rising; the 1990s was the warmest decade in the last thousand years. Sea surface temperatures have increased 0.4-0.8°C (0.7-1.4°F) since the late 19 Century, and over the period 1961 to 2003, global ocean temperature has risen by 0.10°C (0.18°F) from the surface to a depth of 700 m. The world has warmed 0.74°C in the past hundred years and scientists are clear that the world will get warmer this century due to further increases in greenhouse gas concentrations. Global average temperature is forecast to rise 4°C (7.2°F) toward the end of the 21st century."
One of my interesting results from measuring the different heights the water expanded to was that with 1-1/2 tsp's of salt the water expanded more then the cup that had 3 tsp's of salt mixed in the water. It seemed as if the more salt I was going to add to the water the less that it was going to expand when frozen.
I also noticed that it was difficult to measure the different heights that the water's expanded. I did my experiment in clear cups so it was easier to measure the different heights of the frozen water.
Another interesting result I noticed was that it took my cup of water only an hour to completely freeze, while it took my cup with 1-1/2 tsp's of salt about 3 hours, and my 3 tsp's of salt cup took about 4-1/2 hours to freeze.
Relate your thought on this experiment to the scientific question, "is the earth warming?"
"Is the earth warming?" From conducting my experiment with how salt water freezes compared to just tap water, I found that over time temperatures change due to factors that took part in the water, which would be the salt."Just as weather patterns change from day to day, the climate changes too. This occurs naturally, driven by internal and external factors. Through widespread use of land, use of fossil fuels and the building of cities, we have changed our climate." http://www.global-greenhouse-warming.com/climate.html
I was adding a factor to the water which caused the water to take longer to freeze. The more factors we pull into a situation the more "trouble/problems" we may have with the temperatures.
Stated above just shows how with different factors temperature/climate can change. The salt interfered with how fast the water would freeze and just how cold this water would get before it freezes. This is the same for the earth, with cars driving around using fossil fuels and building new cities/buildings also change the temperature and climate on earth. "Temperatures across the globe are most certainly rising; the 1990s was the warmest decade in the last thousand years. Sea surface temperatures have increased 0.4-0.8°C (0.7-1.4°F) since the late 19 Century, and over the period 1961 to 2003, global ocean temperature has risen by 0.10°C (0.18°F) from the surface to a depth of 700 m. The world has warmed 0.74°C in the past hundred years and scientists are clear that the world will get warmer this century due to further increases in greenhouse gas concentrations. Global average temperature is forecast to rise 4°C (7.2°F) toward the end of the 21st century."
Wednesday, January 19, 2011
Homework 1/19-20/11 Stuff around Us?
For your last homework assignment, choose 20 items around you in your world and list their ingredients or materials down to the molecular level. List the chemical names of the ingredients and show a molecular structure image.
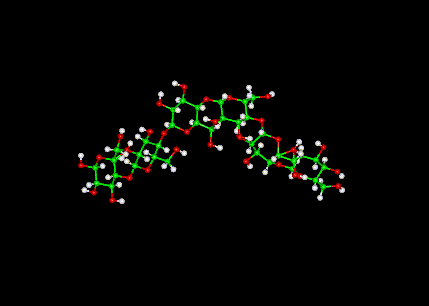
1.) Tums= Calcium Carbonate CaCO3, sucrose C12H22O11, Cornstarch C6H10O5, Talc Mg3Si4O10(OH)2, Mineral Oil(aromatic hydrocarbon-Benzene) C6H6 , natural flavor (peppermint)-Menthol C10H20O, sodium polyphosphate Na5P3O10
 |
| CaCO3 |
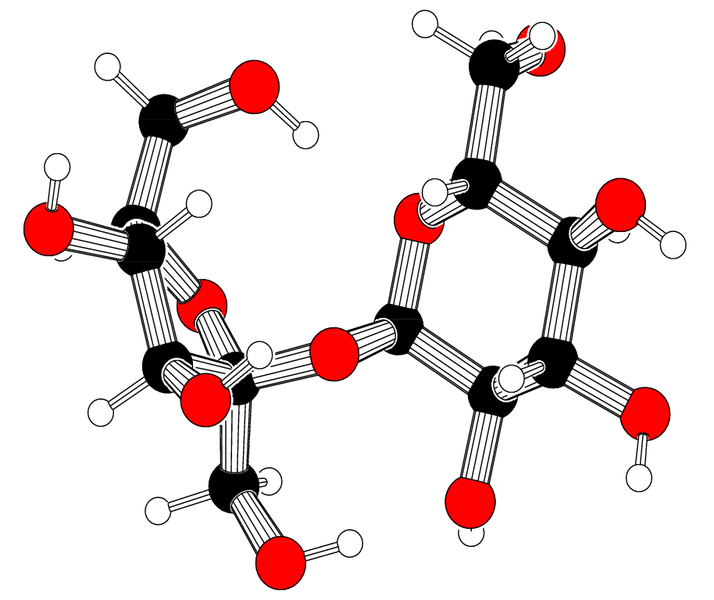 |
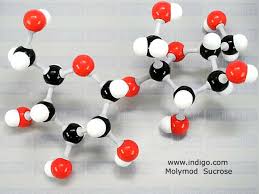
| C12H22O11 |
| C6H10O5 |
3.) Saline Solution= sodium chloride NaCl


4.) Pillow= Polyster (polyethylene terephtalate) (C10H8O4)n

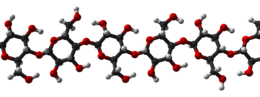
5.) Shirt= Cotton(cellulose) C6H10O5, Spandex (polyurethane) C25H42N2O6

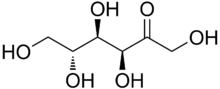















 |
Cotton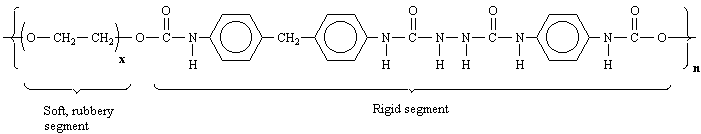 |
6.) Arbor Mist= Pinot Grigo(white grape wine) C2H6O; water H2O; high fructose corn syrup C6H12O6; citric acid C6H8O7; carbon dioxide CO2; potassium sorbate C6H7KO2; potassium benzoate C7H5KO2; potassium metabisulfite K2O5S2







7.) Face Polish= oatmeal, almond, sugar (glucose) C6H12O6
8.) A+D= (active ingredients)Lanolin (wool wax/carboxylic acid) –CO2H, petrolatum

9.) Adderall= dextroamphetamine and amphetamine C9H13N

10.) Microgestin= norethindrone C23H27N

11.) Imitation Butter Flavor: water H2O, propylene glycol C3H8O2, artificial flavor, and FD and C yellow 5.

12.) Cinnamon Sugar: Sugar (sucrose) C12H22O11, cinnamon C9H8O, and cinnamon oil

13.) Ibuprofen: propionic acid C3H6O2

14.) Drain-O= sodium hydroxide HNaO

15.) Nylon- polyamids

16.) Glass- Sillicone dioxide SiO2
17.) Lysol spray: benzalkonium chloride(active)
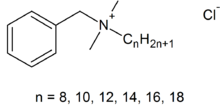
18.) Pop bottle: polyethylene terephthalate (C10H8O4)n

19.) Scarf: acrylic C3H4O2

20.) paper: cellulose (C6H10O5)n

Homework 1/19/11
Use the pH paper to develop a table and graph of the pH of common things around your home.
Think in terms of at least 10 different liquids.
Develop an experiment to see how much anti-acid is needed to neutralize an acidic liquid. Just one liquid.
I had a plastic shot glass and filled it 3/4 of the way with lemon juice. I then added 1/4 of a tum each time until I got one whole tum inside the shot ( I crushed each piece before I put it in, dissolved much faster).
Think in terms of at least 10 different liquids.
Liquid | pH Level |
Vodka | 5 |
Brandy | 5 |
Monster Hitman (energy shot) | 5 |
Contact Solution | 7 |
Hair Spray | 8 |
Almay Make-up Remover | 7 |
Orange Juice | 4 |
Herbal Essences Shampoo | 7 |
Tanning Spray | 7 |
Off- Bug Spray | 8 |
After Bite Itch Eraser | 11 |
Purell Hand Sanitizer | 5 |
Act Mouthwash (alcohol free) | 7 |
Listerine Mouthwash (alcohol) | 7 |
Shout – Stain remover | 8 |
409 | 11 |
Lemon Juice | 2 |
Develop an experiment to see how much anti-acid is needed to neutralize an acidic liquid. Just one liquid.
I had a plastic shot glass and filled it 3/4 of the way with lemon juice. I then added 1/4 of a tum each time until I got one whole tum inside the shot ( I crushed each piece before I put it in, dissolved much faster).
| Amount of Tums- crushed after cut in ¼ ‘s | pH of Lemon Juice—1 sot of lemon juice=2pH |
| ¼ of tum | 3 |
| ½ of tum | 3 |
| ¾ of tum | 5 |
| 1 of tum | 5 |
| 1 ½ of tums | 6 |
| 2 of tums | 6 |
| 2 ½ of tums | N/A |
| 3 of tums | 7 |
Activity 5 1/19/11 pH levels
pH 3 compound to a pH 4 compound.
1.) Which is more acidic?
pH 3 is more acidic.
2.) By how many times?
pH 3 is 10 times more acidic then pH 4.
http://www.elmhurst.edu/~chm/vchembook/184ph.html
1.) Which is more acidic?
pH 3 is more acidic.
2.) By how many times?
pH 3 is 10 times more acidic then pH 4.
http://www.elmhurst.edu/~chm/vchembook/184ph.html
Activity 4 1/19/11 Dissolving
Show an image or animation or description of what is happening when water dissolves NaCl.
The water molecules slowly take apart the Na and Cl from the NaCl.
http://www.northland.cc.mn.us/biology/biology1111/animations/dissolve.html-- really liked this one, describes it while showing an animation.
http://www.chemistry.wustl.edu/~edudev/LabTutorials/Water/PublicWaterSupply/dissolving_movie.html
Why is the freezing point lowered when salt is added to water?
When salt dissolves in water, the Na and the Cl ions leave the salt crystal and mix with the water molecules. Water molecules then form crystals when freezing. The Na and the Cl ions then begin to get in the way of the H2O molecules; which makes it harder for them to become re-arranged into crystals. This then causes the salt water to remain in a liquid state for longer as temperatures reduce.
Why is the freezing point lowered when salt is added to water?
When salt dissolves in water, the Na and the Cl ions leave the salt crystal and mix with the water molecules. Water molecules then form crystals when freezing. The Na and the Cl ions then begin to get in the way of the H2O molecules; which makes it harder for them to become re-arranged into crystals. This then causes the salt water to remain in a liquid state for longer as temperatures reduce.
Subscribe to:
Posts (Atom)

